A new era in cancer therapy dawned in 2011 when the FDA approved ipilimumab (Yervoy) to treat metastatic melanoma. This was the first of a novel class of drugs called immune checkpoint inhibitors (ICIs), which “take the brakes off” the immune system’s ability to kill cancer cells. Today eleven different ICIs are available in the US for more than 40 distinct cancer indications. These therapies can dramatically improve patient outcomes, but typically only in a subset of patients.
FCF’s immunotherapy investments

While enormous investments have been made in cancer immunotherapy, related research for rare malignancies like FLC has significantly lagged that of more common cancers. To help address that issue, FCF has funded a broad range of basic, translational and clinical efforts to support the assessment and development of immunotherapy treatments for FLC.
Those investments fall into four distinct categories:

With the goal of exploring immunological approaches to treat FLC, in 2016 FCF partnered with the Cancer Research Institute (CRI), a visionary nonprofit focused on immunotherapy. Together, FCF and CRI launched a postdoctoral fellowship program for young scientists to initiate FLC-focused projects in the labs of experts in tumor immunology. Six 3-year fellowships have been funded by FCF since the program’s launch. The resulting project have included identifying immune checkpoints biomarkers in FLC, using research models (mouse and zebrafish) to investigate how FLC interacts with intact immune systems, and understanding the characteristics of the T cells that are active within fibrolamellar tumors. Many of the fellows have now progressed to faculty positions and maintain an ongoing research interest in FLC.
In addition, FCF has worked to attract experienced immunotherapy researchers to this cancer and support broad-based collaborations involving many institutions by hosting FLC scientific summits, research working group meetings and other activities.
Most trials of ICIs in liver cancer have focused on conventional hepatocellular carcinoma (HCC) and excluded FLC. Published data on the use of ICIs in FLC are largely limited to case studies and retrospective analyses which indicate that an ICI combined with various other drugs can sometimes induce responses in FLC. One specific therapy that was reported to benefit some patients combined the ICI nivolumab (Optivo) with interferon (a “cytokine,” or modulator of immune signaling) and 5-fluorouracil (a conventional chemo drug). (S. Gottlieb et al., Oncology 99: 310-317 (2021)).
Through a grant approved in 2019, FCF is supporting a clinical trial of this combination therapy at the MD Anderson Cancer Center (Houston, TX), with the goal to understand if and by what mechanisms the different components of the treatment synergize.
The protein coded by the DNAJB1-PRKACA gene fusion drives cancerous growth in nearly every case of FLC. Because this “protein chimera” (designated DP) is found only in the cancer cells, FCF has focused on determining if the DP protein could be exploited to make FLC vulnerable to immune attack by T lymphocytes (T cells). Since 2019, FCF has funded or facilitated the funding of several such research studies. The goal of these investigations has been to lay the groundwork for therapies that could eventually provide patients with armies of “killer” T cells that recognize DP and destroy FLC cancer cells.
These efforts include:
- Developing peptide vaccines. Mark Yarchoan and colleagues at the Johns Hopkins School of Medicine (Baltimore, MD) aimed to produce a therapeutic vaccine to treat FLC by inducing T cells of patients to recognize the DP fusion as “foreign”. Because the junction between the “D” and “P” segments of the FLC chimera is identical from patient to patient, a single vaccine targeting this junction could potentially treat all FLC patients. In a clinical trial funded by FCF, the Johns Hopkins team treated a cohort of patients affected by advanced FLC with a peptide vaccine plus two ICIs (ipilimumab and nivolumumab). In 9 of 12 evaluable cases (75%), the vaccine induced an immune response -the production of large numbers of T cells that were specific to the DP fusion junction. Among these 9 patients, all but one showed some clinical benefit, with a period of non-progression or shrinkage of their FLC tumors. Importantly, in 3 cases the tumors shrank dramatically over time, and the patients achieved deep, durable responses. In one, scans for tumor tissue became completely negative, while in the other two small areas of residual tumor mass were surgically removed. All three of these patients remain in clinical remission today.
In a parallel effort, a group led by Juliane Walz at the University of Tuebingen in Germany independently developed a very similar peptide vaccine. They used it to treat a patient with recurrently relapsing FLC, which was becoming increasingly less responsive to chemotherapy. As with the experience at Johns Hopkins, the patient showed a strong, specific T-cell response to the vaccine. This resulted in a durable remission and the patient currently remains free of detectable disease. The Tuebingen team has also initiated a clinical trial of their peptide vaccine combined with atezolizumab in patients with advanced FLC. (NCT05937295). - Laying the groundwork for adoptive cell therapy. A team in Memphis, TN led by Paul Thomas (St. Jude Children’s Research Hospital) and Scott Strome (University of Tennessee) opted for an alternative strategy to generate the desired army of anti-DP T cells. In efforts partially funded by FCF, they isolated tumor-infiltrating lymphocytes (TILs) from FLC patients to determine if they expressed T cell receptors (TCRs) able to recognize the DP junction. In a paper published earlier this year (A. Kirk et al., Cell Rep. Med.5: 101469 (2024)), they identified one such TCR isolated from a FLC patient. They cloned the gene for that TCR for potential therapeutic use via “adoptive cell therapy.” In this approach, T cells from a patient in need of FLC treatment are genetically engineered to express the same DP-recognizing TCR obtained from the original patient. The recipient patient’s modified T cells would then be expanded in a clinical grade cell culture facility, and then injected back into the patient to carry out their “search and destroy mission” against FLC tumor cells.
As an initial clinical test of this approach, the Memphis team expects to use the first cloned DP-specific TCR gene to build up an army of anti-FLC killer cells for the original donor patient, who had only a small number of T cells bearing that TCR.
While extremely promising, this therapy has a key constraint – the cloned TCR inserted into a patient’s cells must be HLA-type-matched* to a patient’s existing TCRs. The “high affinity” TCR described in the 2024 publication is of an extremely rare HLA type. However, together, the St. Jude team and the Johns Hopkins team are working to identify and isolate a broad range of FLC-specific TCRs from patients who generated an immune response to the peptide vaccine. The hope is that a range of TCRs with a variety of HLA-types can be identified that could be used for adoptive cell therapy for most FLC patients.
While immunotherapy has already changed the landscape of cancer treatment, many patients do not achieve deep, durable responses to the therapies. For example, in the FLC peptide vaccine clinical trial, many patients had an immunological response to the vaccine, but only 25% achieved a lasting clinical benefit. Why is that? What additional factors impact the success or failure of immunotherapy in a particular patient? Important variables probably include: genetic variation, tumor metabolism, actions of immune suppressor cells, immune suppression by the stroma, the microbiome, chemical signals (e.g., cytokines and chemokines), and epigenetic regulation of both tumor and immune cells.
For this reason, many FCF-funded investigations are focusing on defining the metabolism and signaling molecules in the FLC’s tumor microenvironment (TME). The goal is to use that information to increase the percentage of patients for whom immunotherapy proves highly successful.
Investigations currently underway include:
- Defining the single-cell activity landscape of FLC. Like tumors of other cancers, the microenvironment of FLC tumors is highly complex, comprising many different cell types. It is now well-established from investigation of other cancers that cross-talk among different cell types can promote tumor development, growth, and spread. In an effort funded by FCF in 2022, labs at Cornell, Johns Hopkins, and St. Jude are collaborating to develop an FLC tumor “atlas”. This consortium brings together three groups with longstanding interest and experience in FLC research to identify the specific cell types in which FLC genes are active and provide unprecedented resolution of the cellular and molecular landscape of FLC.
- Exploiting the Glutamine Addiction of FLC Cells. Cancer cells’ metabolic activity not only supports the growth of tumors, but it can also subvert the anti-cancer immune response. Many cancers take in and use large amounts of glutamine (an amino acid), both to supply energy and to provide building blocks necessary for cellular growth and proliferation. Some depend so strongly on this amino acid for their survival that they are described as “glutamine addicted.” This utilization of glutamine by cancer cells deprives T cells of the glutamine and oxygen that they also need and dumps toxic metabolites into the TME. As described in a recent “Perspective” story on this website, (https://fibrofoundation.org/attacking-the-metabolism-of-flc/) glutamine-addicted cancer cells may directly compromise immunotherapy. FCF, therefore, is currently funding a clinical trial (NCT06027086) of an experimental new “glutamine antagonist” – DRP-104 – to understand this potential impact. A separate syngeneic mouse model (having a FLC-like tumor and an intact immune system), developed with FCF funding, provided valuable pre-clinical data to support this new trial.
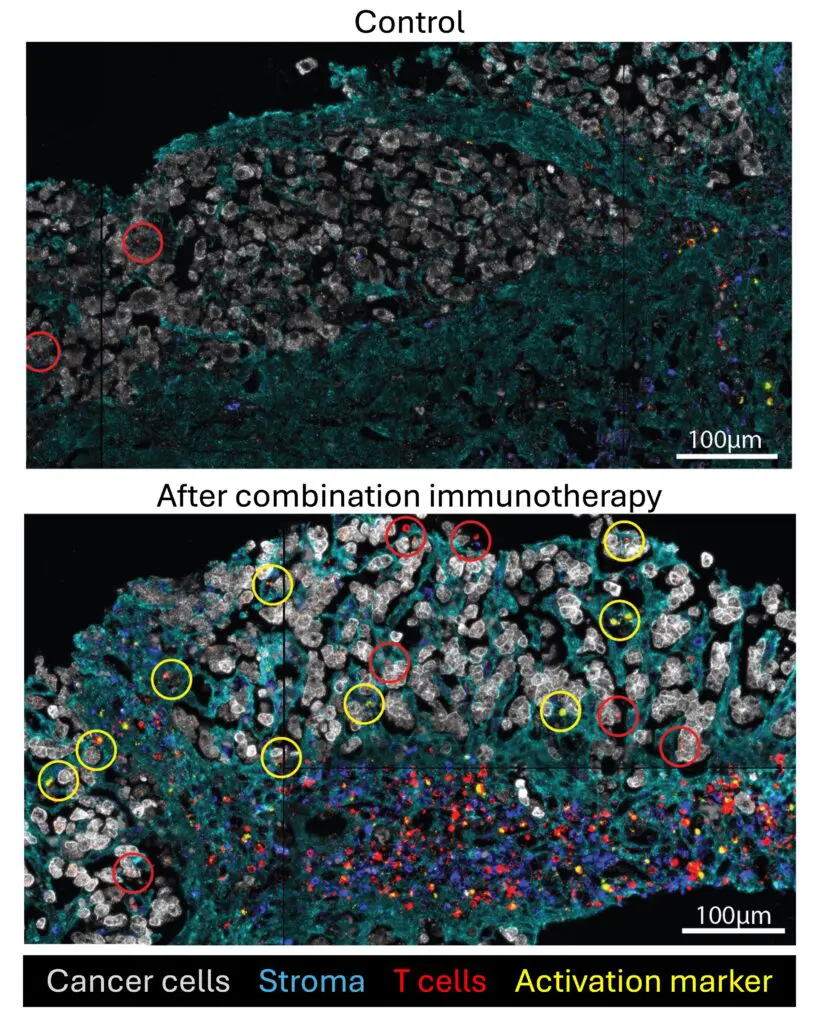
- Activating Anti-FLC T Cells in the TME. FCF grantee Venu Pillarisetty and colleagues at the University of Washington (Seattle) are using cultured slices of fresh FLC tumors to analyze the characteristics of the immune cells present in the TME. Based on this research, they recently reported discoveries that may lead to improved immune treatment of FLC (S. Daniel et al., Sci. Reports 14: 5109 (2024)). In most FLC tumors, the team discovered that T cells had been shut down by factors in the TME. Notably, most of the immune cells were sequestered in the FLC tumors’ characteristic fibrous bands, rather than being close to the actual carcinoma cells.
Could those “inactivated and sequestered” T cells be driven towards the cancer cells? Is the immunosuppressive TME reversible? The team found that combined blockade of PD-1 (an immune checkpoint) and IL-10 (a chemical messenger which helps regulate the activity of the immune system) reactivated T cells in the tumor slices to kill FLC cancer cells! Ongoing studies aim to identify additional immune, cytokine and chemokine, stromal, checkpoint, and immune factors that can modulate the anti-tumor response, with the goal of designing additional ways to augment the immune system’s ability to combat FLC. The team is also planning to identify the FLC tumor antigen(s) recognized by the TILs in their tumor slices, and to determine whether any of the immune cells recognize the DP fusion protein, and especially the junction between this fusion protein’s D and P domains.
Next Steps
While the results of many of these research efforts has been encouraging, much work remains to be done to more fully develop immunotherapy options for FLC.
In addition to driving promising therapeutic approaches into the clinic through sponsored trials, an overarching goal of FCF-funded research is to better understand the immune response to the drivers of FLC and the role of the TME in modulating that response. That knowledge will be critical to optimize immunotherapies against FLC and increase the number of patients who achieve clinical benefit from the approach.
Key questions for investigation include:
- Beyond DP what other specific targets for FLC immunotherapy might be exploited?
- What subtypes of T cells give the strongest and most durable anti-FLC response?
- Beyond killer (CD8) T cells, should additional immune cell types such as CD4 T cells, macrophages, or natural killer (NK) cells be tested for adoptive therapy?
- How important are other immune, cytokine, chemokine, and checkpoint factors to the modulation of the anti-FLC response?
- Would engineering of chimeric antigen receptors against DP or other antigens increase the efficacy and/or availability of targeted immunotherapy for FLC patients?
- How many TCRs matched to different HLA genes and alleles should be collected to optimize outcomes and maximize access to adoptive cell therapy for FLC patients?
- How much do ICIs contribute to successful therapy? In which patients should they be used?
More broadly, two further pressing questions are:
- What other treatment modalities might be combined with immunotherapy to improve recurrence-free survival and overall survival in FLC?
- Under what circumstances are immunotherapy approaches best used in FLC? For example, what are the relative strengths and weaknesses of vaccination versus adoptive cell therapy approaches for patients at different stages of disease? Can vaccination of apparently disease-free patients after surgery be used as adjuvant treatment to prevent future recurrence of FLC?
Research funding note
While funding research for rare diseases is always an issue, FCF has been thrilled that many of its “high risk, high reward” investments have provided research teams the data they needed to secure subsequent investments from other large funders, including the National Institutes of Health/National Cancer Institute (NIH/NCI) and CRI. That external funding frees FCF to fund other “earlier stage” research.
Cancer research funding is quite scarce, especially for rare diseases. For example, in 2023, the NIH only funded the top 12% of all its applications for large grant awards (R01). That means that researchers need a “nearly perfect” application to ensure funding from the largest funder of cancer research in the US. As a result, FCF’s ability to fund and drive research focused specifically on FLC is absolutely critical to unlocking the mysteries of this disease and identifying new, effective treatments!
Recent funding “big wins” in immunotherapy that have been supported by earlier FCF-funding include:
- In 2022, NCI awarded a multi-year multi-million dollar R01 grant to St. Jude and Johns Hopkins to identify and isolate FLC-specific TCRs from immunologically responsive patients from the peptide vaccine trial.
- In 2024, large additional grants were awarded to Johns Hopkins to support the glutamine antagonist trial that FCF began funding in 2023, including a $1.0 million Clinical Innovator grant from CRI and a multi-million dollar R01 grant from NCI.
*HLA typing, also called tissue typing, tests for proteins on most cells of the body that vary from person to person and are important to immune function. They help to identify the difference between self and non-self.

