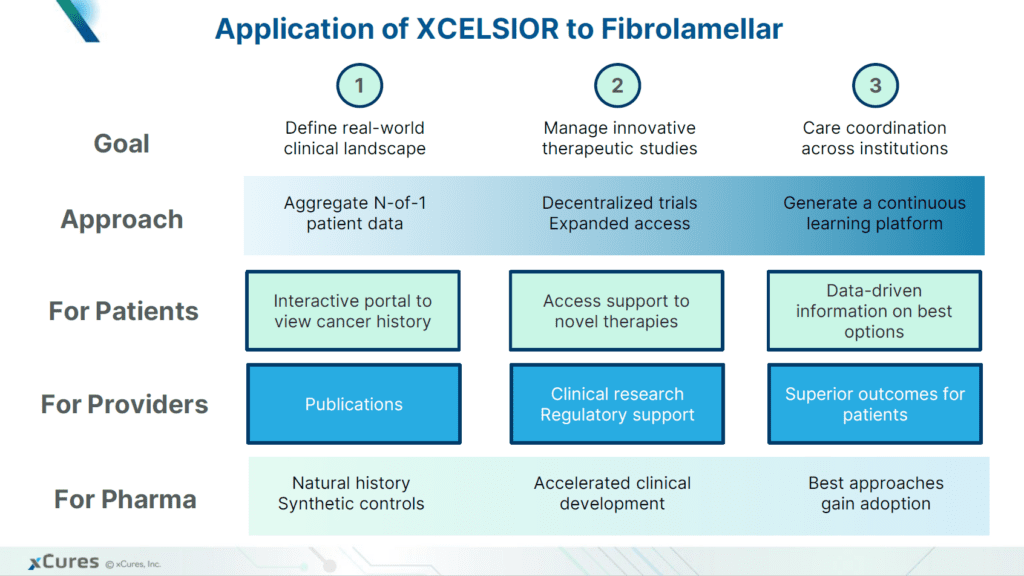
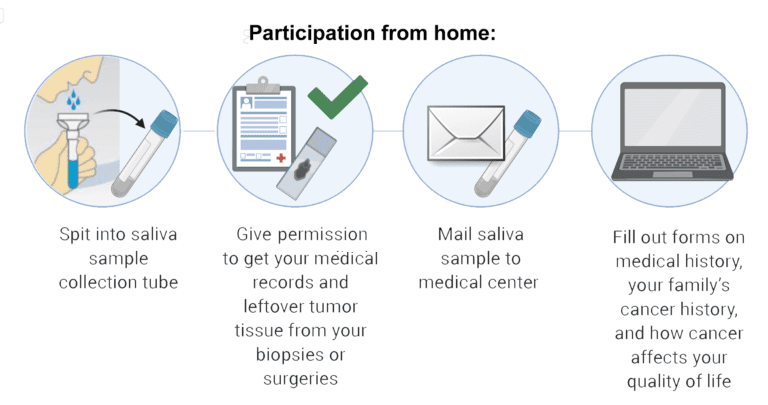
Development of successful new treatments for fibrolamellar will require a deep understanding of the causes and progression of the disease. Since FLC patient populations are so small and dispersed, information about their treatments and outcomes is incomplete. Systematic data gathering efforts, such as registries and natural history studies, can play a major role in closing critical gaps in our knowledge of the disease.
Increasing the number of participants involved in these data studies increases the accuracy and power of the results. Every patient’s information is important, so please participate if you can!
What is a patient registry?

Patient registries are systems for collecting, storing and analyzing a uniform set of information about patients for research or clinical purposes. Most patient registries collect a defined set of demographic, epidemiological, quality of life, treatment, and clinical data. That data can be collected through patient-reported surveys or extracted directly from medical records. Since most registries are broad in scope, registry studies can help researchers collect disease information, consider different research hypotheses, identify patient outcomes and study best practices in treatment. Data that a registry shares with researchers is “de-identified” to preserve the privacy of the participants.
What is a natural history study?

Some use the terms registry and natural history study interchangeably. However, unlike registries which store a broad range of data that can be used to answer many different research questions, natural history studies are designed with a very specific goal – to track a disease’s course over time by identifying factors such as demographics, environmental conditions, and genetic variables that correlate to the disease and its outcomes. These studies can produce greater understanding of how a disease like FLC develops and how it can be treated most effectively.
Why are registries and natural history studies important for fibrolamellar research?
Registries and natural history studies can help accelerate FLC research in many ways. Critically, they provide researchers a set of consistent, comparable data from a large group of patients to study. Analyzing such data can help FLC researchers build a more comprehensive understanding of the disease, advance research hypotheses, determine patient outcomes, and identify potential best practices in treatment. The information can help answer questions such as:
- Is the disease more common in particular demographic groups?
- What treatments are being administered? How is that changing over time?
- How do patient outcomes vary? Why?
- How does the FLC develop and progress? Are there different disease subtypes?
Registries and natural history studies can also reduce the time and cost of individual research efforts, especially if they offer open access to relevant data. In addition, the “real world evidence” obtained from a natural history study could potentially provide a baseline to drive the approval of new drugs or therapies that demonstrate better results. This could be extremely important for extremely rare diseases like FLC where the inclusion of a placebo control arm in clinical trials may never be feasible.
The Fibrolamellar Cancer Foundation does not directly fund or manage any registry or natural history studies for FLC. We are thrilled that several other organizations are systematically gathering patient data and are making that data accessible to all FLC researchers. FCF collaborates with all these research data providers and strongly encourages FLC patients to participate in as many of these initiatives as possible.
Current fibrolamellar data initiatives include: