In this brief video, Dr. Mary Frances Wedekind explains how fibrolamellar patients would go about enrolling in the study. In addition, below are a series of common questions about the National Cancer Institute’s Natural History Study of Rare Solid Tumors and its importance to research on fibrolamellar carcinoma (FLC).
The NCI Natural History Study of Rare Solid Tumors studies people with rare tumors over time. The goal of this study is to collect information from rare tumor patients to learn how each tumor type grows and develops. This will help researchers understand these rare tumors better so that they can develop new treatments.
One of the reasons that natural history studies are so important to rare diseases like FLC is that they help the disease to be better understood. A natural history study examines the characteristics of the disease, assesses how it progresses over time, and potentially identifies patterns that might otherwise go unnoticed. A longitudinal natural history study can track the complete course of the disease from its onset. It is intended to describe all aspects of the disease and identify the demographics, genetics, environmental impact, and other variables that correlate with the disease and outcomes. Natural history studies may also collect patient reported outcomes (PROs), which provide insight into how the disease and its progression impact different aspects of the patient’s life.
Once significant natural history study data is available for a disease like fibrolamellar carcinoma, it can help with patient care. It can help identify the best practices of patient care as well as point out key research priorities. It can potentially identify meaningful biomarkers. Information obtained from a natural history study can also play an important role in drug development, from drug discovery to the design of clinical studies intended to support a drug’s approval. Patient reported outcome measures are important for determining the success of clinical trials; the significance of natural history PRO data cannot be over emphasized. Without a natural history study in place defining the progression of fibrolamellar, it could be difficult to determine how any new therapy has changed the disease progression. In addition, natural history study data can potentially be used to support regulatory submissions in rare diseases like FLC, since patient populations are small and the opportunity to include a control group in trials may be limited.
A natural history study looks at the changes caused by a disease over time in a patient population. The importance of the natural history is that it provides researchers with a baseline to examine how the disease progresses so that future studies of treatment options can determine if the treatment is effective. In some cases, natural history study data could potentially serve as the ‘control group’ for a clinical trial. It may also allow researchers to see previously unforeseen patterns in the disease progression.
Because it is a study, the results of a natural history study will be compiled, published and shared.
On the other hand, a registry is not a study. It is a set of data. A natural history study usually has a start and stop point and a defined set of questions asked and analyses conducted. A registry is continuous. The types of information in the registry may sometimes be the same as that in a natural history study, and a natural history study often uses data from a registry, but a registry is not a study. It is a data set that could be used to conduct studies, including those that assess natural history, diagnosis, treatment, quality of life, healthcare providers, clinical reporting, clinical testing, etc.
This NCI study offers a unique combination of value to research and potential value to participating patients themselves.
Benefits to research: Patients who take part in the study may help speed up the development of new treatments for fibrolamellar by helping researchers learn more about why and how fibrolamellar progresses. The analysis of the clinical and biospecimen data from this natural history study may allow researchers to gain new insights into fibrolamellar biology and develop treatments tailored to the disease. Additionally, approval of new treatments for FLC will require a demonstration that they change the natural history. The baseline data from this study may make it possible to meaningfully assess the effectiveness of new treatments in clinical trials.
Benefits to patients: In addition to aiding research, participating patients can also receive treatment perspectives from the NCI clinical team through the study’s optional clinical component. Because the NCI clinical team reviews the medical records collected and the study includes some CLIA-grade testing suitable for diagnosis, the team can give participating patients, at their request, treatment recommendations and information about clinical trials they may be eligible for. As part of that process, the NCI clinical team is happy to provide patients with “second opinions” on proposed care plans.
Importantly, there are no direct costs to patients for participating in this study. If patients choose to receive any care or treatment perspectives from the NCI clinical team through the study’s optional clinical component, that care will be performed at no cost to the patient (or the patient’s insurance) as long as the patient is enrolled in the study. In addition, if an enrolled patient is invited to travel to the NIH Clinical Center, the study will cover the patient’s travel costs for the visit, including airfare, airport transportation, reasonable hotel accommodations and meal vouchers.
FCF believes that the NCI study is an important initiative for the fibrolamellar community to support for several reasons. Because it is an established study, it allows a faster implementation than developing a new independent study. In addition, it will
- Provide the opportunity to involve researchers from the National Cancer Institute, a world-class research institution, directly in fibrolamellar-related research
- Leverage existing federal funding from the “Cancer Moonshot” initiative, increasing the total research funding focused on fibrolamellar
- Offer direct benefits to participating fibrolamellar patients, including:
- Notifying the patient about any clinically-actionable mutation information that may be discovered from the study’s genetic testing
- Providing care or treatment perspectives from the NCI clinical team through the study’s optional clinical component.
- Giving second opinions on treatment plans to any interested patients.
- Not charging either the patient or their insurance company for any care and consultation a patient receives from the NCI clinical team
- Covering a patient’s travel costs if an enrolled patient is invited to travel to the NIH Clinical Center.
- Enable significant leverage and operational efficiencies, since many elements of the natural history studies will be shared across many rare cancer types
- Incorporate testing for the fibrolamellar fusion gene.
The Natural History Study of Rare Solid Tumors is not a treatment trial, so it is not testing whether an experimental treatment will work or if it is safe. Because you are not exposed to an experimental treatment, natural history studies are considered very safe and have very minimal risks.
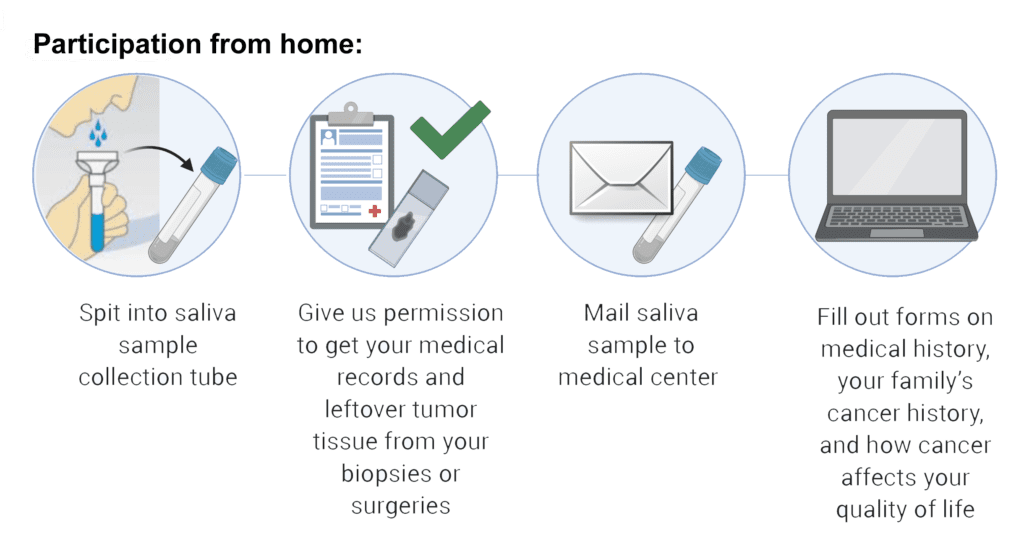
NCI will collect medical records and imaging from you or your physician to be reviewed by the study team. They will also collect samples of your tumor that may be available from a prior biopsy or surgery. You will be asked to send a saliva sample to NCI. Additionally, you will be asked to fill out questionnaires about your medical history, your family’s medical history, and how having a rare tumor impacts your life, all of which you can do online from home or through paper forms the NCI can mail to you. Based on a review of your medical records, you may be invited to the NIH Clinical Center to meet with experts who will perform additional tests and answer questions. You may also be invited to participate in focus groups about how living with a rare tumor affects your life, but you do not have to participate in these groups. All participants will receive a follow-up form from NCI approximately once a year to record any changes in their health.
Your saliva and tumor samples will be used to perform genetic testing. NCI will combine your data with data from other people with fibrolamellar. If they collect blood (this will only be done for participants who are invited to come to the NIH Clinical Center), they will look at what kinds of cells and molecules are in your bloodstream that might affect your tumor. They will also look at immune cells in your tumor samples. Your samples may also be stored for future research. NCI may share the information they learn with other scientists while protecting your privacy to allow many researchers to work on new treatments for fibrolamellar.
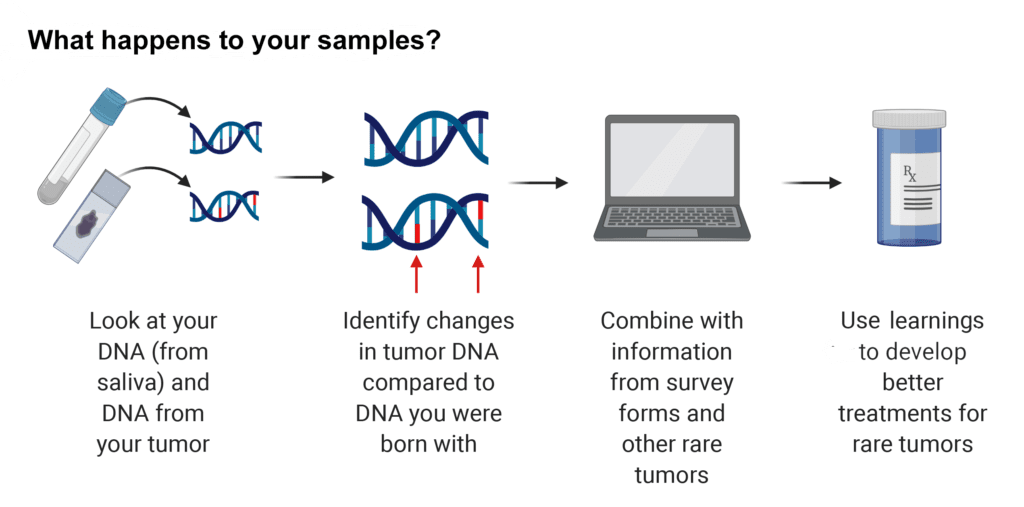
Some of the results will be returned, but some will not. The NCI study team will test your tumor for common cancer mutations and the results may suggest drugs that could treat your tumor. If the study team suspects that cancer may run in your family, they may test your blood or saliva for mutations, as well. If any clinically actionable mutations associated with your disease are identified in your tumor samples, the NCI study team will contact you and your doctor to share those results.
Some of the genetic testing the NCI study team will perform is done for research purposes and is not the same as having genetic testing ordered by a doctor from a clinical laboratory. If new mutations are identified through research-grade testing, that information would be included in the published results of the study, but the results from your tumor alone will not be shared with you.
The study will not charge you or your insurance company for any genetic testing that is done.
Yes. You can continue to receive treatment for fibrolamellar while participating in the Natural History Study, but you will not receive treatment through the study itself since it is not a treatment trial. You can continue to receive any care recommended by your medical providers or participate in any ongoing clinical trial. If the study team is aware of any clinical trials you may be eligible for, they will help you learn more about those trials.
For more information about trials enrolling fibrolamellar patients, visit FCF’s clinical trials page.
No. Participants can provide saliva samples and other information from home. However, based on a review of your medical records, you may be invited to the NIH Clinical Center at a future date to meet with experts who will perform additional tests and answer any questions you may have.
There are no direct costs to patients for participating in this study. If a patient who is enrolled in the study is invited to travel to the NIH Clinical Center, the study will cover the patient’s travel costs for the visit, including airfare, airport transportation, reasonable hotel accommodations and meal vouchers. In addition, if patients choose to receive any care or treatment perspectives from the NCI clinical team through the study’s optional clinical component, that care will be performed at no cost to the patient (or the patient’s insurance) as long as the patient is enrolled in the study.
This study is open to broad participation by fibrolamellar patients, with priority enrollment given to patients diagnosed at age 39 or younger. In order to perform the planned genomic testing, patients would ideally have had surgery to remove their tumor within the last 5 years, since degradation of older tissue can limit its usefulness for certain types of genetic analysis.
The NCI study and the FCF BioBank are separate and distinct initiatives. Participation in both projects is possible and encouraged. Patients can enroll in the NCI natural history study whether or not they have previously contributed tumor tissue to the FCF BioBank. If you have already contributed tissue to the FCF BioBank, Patty Cogswell, our Repository Manager, will contact you about having tissue sent to the NCI study on your behalf. We want to simplify and streamline the tumor donation process for fibrolamellar patients across all research initiatives. If you have surgery after beginning participation in the natural history study, tissue delivery to both initiatives can easily be coordinated.
One of the key roles that the FCF BioBank plays is to make FLC tumor samples available to all researchers of the disease. The FCF BioBank has also been working closely with organizations such as the Broad Institute and Harvard University to use tissue donations to create tumor cell lines (cancer cells grown in petri dishes), organoid cultures (groups of cancer and supporting cells grown together) and xenograft models (human cancer cells grown in special mice that lack immune systems). Each of these types of cancer “models” has strengths and weaknesses, but all are crucially needed by research teams to understand fibrolamellar carcinoma biology and identify new treatment strategies.
To learn more or to enroll in the study, you can:
- Visit www.cancer.gov/mypart, or www.clinicaltrials.gov/ct2/show/NCT03739827 to learn more about the study details
- Email NCICCRRareTumorClinic@mail.nih.gov to ask specific questions to the study team, or begin the enrollment process
- Email info@fibrofoundation.org to ask FCF more about its sponsorship of this study.
In addition, FCF is planning to host a virtual question and answer session with one of the leaders of MyPART where you can ask your questions directly to a member of the study team. Please contact us at info@fibrofoundation.org if you are interested in attending that event once it is scheduled.
