Cancers grow through uncontrolled cell proliferation. Repeated rounds of division of single cells to yield “daughter” cells cause tumors to expand exponentially. Cancer therapy aims not only to slow down or prevent cell division, but also to cause the malignant cells to die. Efficient killing is crucial because even a tumor that is barely detectable on a radiological scan contains several million cancer cells, each one potentially capable of causing a lethal relapse.
Some cancers are highly susceptible to certain chemotherapy drugs, while others are intrinsically less sensitive. Unfortunately, fibrolamellar carcinoma (FLC) often responds poorly to chemotherapy. Complete remissions after chemotherapy treatment are rare, although large primary tumors may shrink sufficiently to enable surgical removal, and localized areas of metastatic disease may disappear from scans, at least temporarily. More effective “systemic” therapy of FLC (treatment delivered to the entire body) is urgently needed.
A soon-to-open clinical trial (clinicaltrials.gov identifier NCT06620302, “Testing the Addition of an Anti-cancer Drug, DT2216, to the Usual Chemotherapy Treatment for Relapsed or Refractory Solid Tumors and Fibrolamellar Carcinoma”) offers a novel approach to FLC treatment. It will test the combination of the anticancer drug irinotecan (brand name Camptosar) with DT2216, an experimental drug designed to overcome a major mechanism through which cancer cells may resist chemotherapy. Patients will be able to enroll at multiple hospitals affiliated with the Children’s Oncology Group (COG). The study is led by pediatric oncologists Dr. Michael Ortiz (Memorial Sloan Kettering Cancer Center) and Dr. Allison O’Neill (Dana-Farber Cancer Institute). The trial is expected to be activated on May 27, 2025, and enroll its first patients shortly afterwards.

The Fibrolamellar Cancer Foundation is supporting the trial by providing Dialectic Therapeutics (Dallas, TX) the funding required to manufacture and supply the experimental drug, DT2216. Dialectic Therapeutics is a Texas-based clinical stage biotechnology company focused on creating innovative new cancer-treating compounds that selectively cause cancer cells to commit suicide or become more susceptible to chemotherapy. DT2216, Dialectic’s lead product candidate, is currently being investigated as a single agent and as part of a combination therapy in several liquid and solid cancer tumors.
Rationale for the trial
Classic chemotherapy drugs kill actively proliferating cells, whether cancerous or not. Many normal cells in the body are “mature,” meaning they no longer divide, but carry out specialized functions. Such cells are usually much less sensitive to chemotherapy than cancer cells. However, some normal cells turn over frequently, such as red and white blood cells, cells that line the gut, and those of hair follicles. A steady supply of new specialized cells in the high turnover tissues derives from pools of immature “stem and progenitor” cells which divide frequently. Like cancer cells, these immature cells are generally susceptible to chemotherapy. This leads to well-known side effects of many anticancer drugs, such as anemia, a weakened immune system, severe intestinal irritation, and baldness, which limit the doses at which the drugs can be administered safely. A chemo regimen can succeed only if cancer cells are more susceptible to the therapy than normal cells.
The idea of using a combination of irinotecan and DT2216 for FLC treatment emerged from drug repurposing studies conducted by Dr. Sanford Simon (Rockefeller University) and colleagues several years ago. They first screened a collection of more than 5,000 compounds for the ability to kill fibrolamellar cells using six FLC tumor lines derived from patients and grown in special mice. (These are called “patient derived xenografts,” abbreviated PDX.) The test chemicals included virtually all drugs approved by the Food and Drug Administration (FDA) for any medical use, plus many earlier stage drugs, especially those known to be active against cancer cells. The screen focused specifically on the ability of the drugs to kill FLC cells via a dramatic process called “apoptosis” or “programmed cell death.”
Apoptosis is essentially organized cellular suicide driven by the activation of powerful degradative enzymes that break down proteins and DNA, leading to cell shrinkage, membrane “blebbing,” fragmentation, and packaging of the remains in neat bundles to be gobbled up by wandering phagocytic cells.
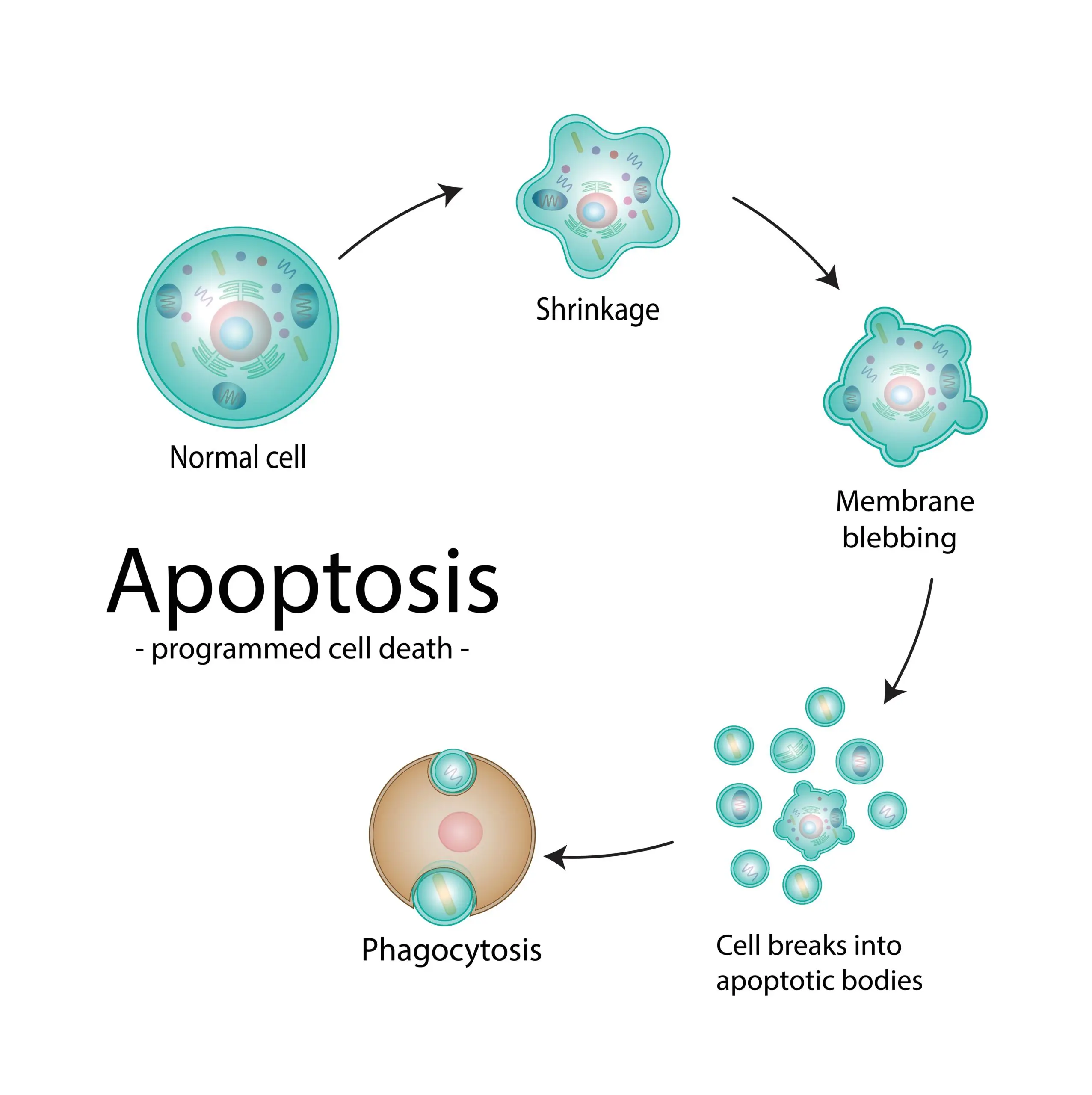
Apoptosis is frequently induced by a major threat to a cell’s genetic integrity. It can occur in response to DNA damage by radiation or chemicals, interruption of DNA replication, or disruption of the segregation of replicated chromosomes into daughter cells during cell division. Irinotecan interferes with DNA replication by blocking the activity of Topoisomerase I (Topo I), an enzyme that untwists the double helical DNA chain to enable copying of both strands of the long DNA molecule present in each chromosome. TopoI inhibitors cause the DNA chains to break during replication, which signals cells to initiate the apoptotic death program.
The Rockefeller team observed that FLC cells were broadly resistant to chemotherapy, as well as to various “targeted” anticancer drugs aimed at specific signaling processes that drive the uncontrolled growth of many cancers. Irinotecan (more precisely, an active metabolite of the drug made in the body) was one of a small set of drugs that showed significant ability to kill cells of the PDX models and of cancer cells taken directly from FLC patient biopsies.
The investigators also observed killing of FLC cells by drugs that targeted a set of related proteins known to protect cells against apoptosis. This protein family is called BCL, because the first example was discovered in B-cell lymphomas. The key anti-apoptotic protein in FLC turned out to be BCL-xL, an “extra-large” family member. Inhibitors of BCL-xL showed some killing activity against FLC, while drugs selective only for BCL-2, another important family member, were not effective. Furthermore, irinotecan was most active against FLC cells with the lowest levels of BCL-xL, and less effective against those with higher levels of this protein. This prompted testing of combinations of irinotecan with several inhibitors of BCL family proteins. Irinotecan and an inhibitor with strong activity against BCL-xL (navitoclax) synergized to induce apoptosis.
DT2216, Dialectic’s improved drug against BCL-xL
The use of BCL-xL inhibitors like navitoclax in cancer patients has been hindered by an important limitation – BCL-xL is critical for the survival of platelets, which are important for blood clotting. Most of the available inhibitors caused the population of platelets in the blood to plummet to dangerously low levels (a condition called thrombocytopenia).
To overcome this problem, Guanrong Zheng, PhD (Professor of Medicinal Chemistry at the University of Florida and Scientific Co-Founder of Dialectic Therapeutics) designed a new BCL-xL inhibitor. He took advantage of an emerging technology to create drugs called PROteolysis Targeting Chimeras (PROTACs), or protein degraders. Rather than inhibiting the activity of a target protein, a PROTAC binds to that protein and attaches a signal that causes it to be transported into the proteasome, a cell’s machinery to breakdown defective protein molecules and those it no longer needs. It’s like slapping a tag on the protein that reads “throw me in the garbage.” To generate a drug that would work in cancer cells but would spare platelets, Zheng’s group started with a molecule that can bind to BCL-xL in any cell. However, they attached to it a degradation tag that works in cancer cells but not in platelets, taking advantage of a small difference in how these cells label the proteins aimed for destruction.
Preclinical studies have shown that DT2216 is a highly effective treatment for several liquid and solid tumors as a single agent and in combination with chemotherapy. Dialectic Therapeutics has also completed a Phase 1 first-in-human study of DT2216 (as a single agent) in patients with various cancers. This trial established DT2216’s safety and tolerability, and it confirmed that the drug does not cause thrombocytopenia.
Pre-clinical testing of DT2216 in FLC
Subsequent tests of DT2216 in combination with irinotecan against human FLC cells gave promising results, which were published in 2022 by Dr. Simon and colleagues. While there was some variation among FLC lines, correlating with their different levels of expression of BCL-xL, the data clearly confirmed synergy of the two drugs. To predict how the combination would work in patients, the investigators used human FLC cells grown in mice (PDX models) with several cycles of drug treatment followed by a post-treatment period without drug. In a representative test, irinotecan alone halted growth of FLC tumors during the treatment cycles. However, during the post-treatment period the tumors immediately resumed their initial rapid rate of growth. By contrast, the combination of irinotecan and the DT2216 caused substantial reductions in FLC tumor mass. Moreover, the benefit persisted well after the end of the drug treatment, although tumor growth eventually began again in most of the mice.
New trial design
On the basis of these findings, Dr. Michael Ortiz (Memorial Sloan Kettering Cancer Center) and Dr. Allison O’Neill (Dana-Farber Cancer Institute) began developing the new Children’s Oncology Group clinical trial. Once open, patients will be able to enroll in a broad network of 21 sites within the NIH’s Pediatric Early Phase-Clinical Trial Network (PEP-CTN). The team believes that in many patients the DT2216 / irinotecan drug combination may be capable of inducing partial remissions of significant duration. In the context of a cancer that historically has proven resistant to chemotherapy, this would represent an important clinical advance.
The resulting new COG clinical trial plans to test the safety, side effects and best dose of DT2216 in combination with irinotecan. The initial Phase 1 portion of this study will be carried out in patients between ages 1 and 21 years who have any type of solid tumors that have shown resistance to other treatments. This phase will include FLC patients; however, many subjects may have other cancers. Its main goal will be to determine a recommended Phase 2 dose of DT2216, administered in combination with a standard dose of irinotecan. It will also assess how DT2216 is absorbed, distributed, altered, and excreted by the body, as well as any toxicities. Finally, this component of the study will assess, to the extent possible, the anti-tumor activity of the drug combination in the tested patients. Between 6 and 24 patients will be required to complete the dose escalation part of this trial.
The Phase 2 portion of the study will be carried out exclusively in FLC patients up to 39 years old. DT2216 will be administered at the recommended dose established in Phase 1, together with irinotecan at its standard dose. Its main goal will be to define anti-tumor activity of the DT2216/irinotecan combination in FLC patients. Between 8 and 15 patients can be enrolled in this phase.
More information about the clinical trial, including specific inclusion and exclusion criteria is available here. Enrollment in both phases of the trial is limited, so please reach out to your care team if you are potentially interested in participating.
If this trial proves successful, it should open the door to determine if DT2216 synergizes with other therapies. If DT2216 is shown to drive the “assisted suicide” of FLC, this clinical study could provide a valuable new approach for the treatment of this cancer.