
To accelerate progress towards a cure, the Foundation, working with its Medical & Scientific Advisory Board and other researchers, prioritizes research efforts that are most likely to lead to the development of effective systemic therapies for the disease. These include building the critical “enabling” infrastructure necessary to efficiently support FLC research efforts, driving basic research that closes gaps in our knowledge about the pathways involved in the growth and survival of FLC tumors, and investigating the promise of new targeted therapy and immunotherapy approaches. All are critically important to making sustained progress against this disease
Currently, FCF is focused on 5 specific area of research, as listed below. Click on each area for more information:
Improved epidemiological data for FLC is critically needed. Published estimates of the incidence of FLC vary by more than an order of magnitude, affecting the perception of the feasibility of clinical trials, the financial support for research, and industry investment in the development of therapeutics. Based on data collected by the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program, the incidence of FLC in the United States is estimated at about one in 5 million person-years, or about 60 new cases per year. However, other published studies indicate that the SEER result may be an underestimate, and it is possible that there are several hundred new cases annually in the US alone. Generating a better understanding of the actual incidence, demographics and experiences of the patient population would help efforts to develop improved therapies for FLC.
Click here to view the epidemiology-related projects FCF has funded.
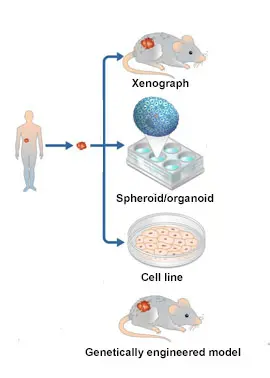
Model systems are vital to biomedical research. While studying cancer in humans is extremely important, model systems allow scientists to make biological discoveries and test potential therapeutics in a pre-clinical setting, bridging the gap between clinician scientists and basic researchers. Experimental systems for studying human cancer include cancer cell lines, 3D organoids, genetically engineered mouse models, and patient-derived xenografts (PDXs). These models form the basis to investigate the biochemical or genetic pathways of cancer and can be used as platforms for drug screening and therapeutic discoveries.
Within the last decade, tremendous progress has been made in developing the first model systems for FLC. However, the paucity of cancer models is a big issue. Consequently, developing additional models, improving existing ones and making them accessible to the entire research community are critically important to sustain research progress. The FCF Biobank can provide a valuable source of tumor material for patient-derived model development efforts.
Additionally, new diagnostic tools, such as detection of the DNAJB1-PRKACA fusion in circulating tumor DNA, should also be sought in order to enable earlier identification of patients with FLC, better tracking of responses to therapy, and better assessment of the level of patients’ residual disease.
Click here to read about the model development projects FCF has funded.
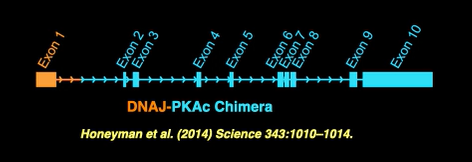
The discovery of the DNAJB1-PRKACA fusion was an important milestone in our understanding of the disease. However, further research is needed to develop successful new therapeutics for FLC.
A major priority of FCF is supporting research initiatives that could lead to the development of targeted therapies. These include efforts centered on targeting the DNAJ-PKAc fusion protein itself, as well as others focused on the “downstream” signaling pathways controlled by that cancer driver. In all cases, functional studies are essential to identify the key genes and biochemical pathways on which the FLC cells depend for survival and growth. To do that, understanding how DNAJB1-PRKACA leads to FLC, determining exactly how other molecular pathways are dysregulated in the disease, and defining potential molecular targets by studying genes and proteins preferentially expressed by FLC cells are all extremely important. In addition, research advancing our understanding of FLC cell biology and metabolism, as well as the factors that contribute to metastasis and heterogeneity within tumors and among patients remain essential.
Once specific pathways and potential targets are defined, candidates for FLC therapeutics can emerge by testing known inhibitors of the defined molecular targets, and from broader screens of chemical compounds.
Click here to view descriptions of the projects FCF has funded in this area.
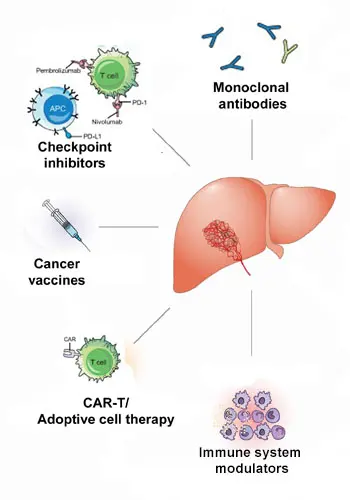
Harnessing the immune system to treat cancer has revolutionized cancer treatment in recent years. Despite FLC’s relatively low tumor mutational burden, immunotherapy of FLC appears feasible.
However, to develop such immunotherapy approaches for FLC, a range of foundational research is required. Understanding the interactions between FLC tumors and the immune system, the details of the tumor microenvironment, as well as specific immune checkpoint markers for FLC will be critically important. In addition, the identification of unique cell surface markers of FLC could potentially provide a basis to develop therapeutic monoclonal antibodies and antibody-drug conjugates, as well as adoptive cell therapies.
Focus areas for immunotherapy-related research in FLC include:
- Use of immune checkpoint inhibitors (ICIs) to block the “checkpoint” signals that cancer cells often produce to turn off the immune response to the tumor.
- Development of therapeutic vaccines to stimulate the immune system to recognize FLC tumor cells as “foreign”. Peptides from the DNAJ-PKAc chimera could serve as a such a target for specific immune attack.
- Investigation of adoptive cell approaches to make a patient’s own immune cells better able to attack the cancer. This approach focuses on genetically engineering the immune cells of a patient to recognize a specific target expressed in the cancer cells, expanding the number of those engineered cells in culture, and then returning an “army” of the resulting anti-cancer killer cells to the patient’s body.
- Modulation of the tumor environment to counteract some of the tumor’s defenses and help make the immediate environment around the tumor more conducive to an immune response.
Click here to read descriptions of the immunotherapy-related projects FCF has funded.

Currently, there are few effective treatments for FLC aside from surgical resection and no clinically-proven systemic therapies to manage advanced disease. Since a significant number of FLC patients have metastatic disease at the time of diagnosis, new therapeutics are urgently needed for FLC.
The Foundation is committed to supporting the development of the clinical trials necessary to bring new therapies to FLC patients and support the development of an evidence-based standard of care for the disease.
This includes supporting well-justified clinical trials that advance promising new therapies, in addition to testing “repurposed” drugs that have already proven effective in other diseases.
Click here to view the active and completed clinical trials FCF has funded.
